
Home | Group | Research | Publications | Seminars/Workshops | Teaching | Open Positions | How to find us | Intranet
Deutsch | English

Home | Group | Research | Publications | Seminars/Workshops | Teaching | Open Positions | How to find us | Intranet
Deutsch | English
The penetration depth of low-energy electrons into a material is limited. They are thus an ideal tool for the chemical modification of surfaces and can be used to initiate reactions leading to surface functionalisation.
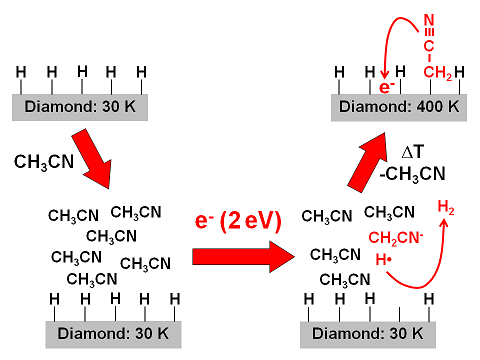
Electron attachment can initiate reactions that link organic molecules to a material. For example, if a thin layer of acetonitrile is condensed onto a hydrogen-terminated diamond surface and exposed to electrons at suitable energy, CH2CN fragments are linked to the diamond.
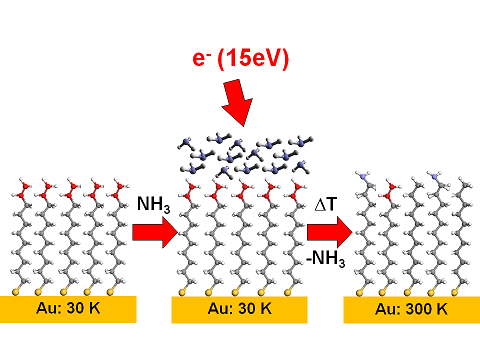
An electron-induced coupling of ammonia to a CC double bond can also be used to functionalize a self-assembled monolayer (SAM). In this process, ammonia also acts as reducing agent and protects the SAM against radiation damage.
Plasmas are widely used in processes for surface treatment. They contain various reactive particles including free electrons. We have investigated, within a joint project with the IFAM, the role of low-energy electrons in plasma processes used to fabricate novel functional siloxane coatings.
![]()
More information:
(1) Electron-Induced Chemical Reactions for Surface Functionalization;
P. Swiderek, in: Wandelt, K., (Ed.) Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry, vol. 4 (2018), pp 702–710.
(2) Functionalisation of a self-assembled monolayer driven by low-energy electron exposure;
T. Hamann, L. Kankate, E. Böhler, J.-H. Bredehöft, F. Zhang, A. Gölzhäuser, P. Swiderek, Langmuir 28, 367 (2012).
(3) Modification of polydimethylsiloxane coatings by H2 RF plasma, Xe2* excimer VUV radiation, and low-energy electron beams;
P. Swiderek, E.Jolondz, J.H. Bredehöft, T.Borrmann, C.Dölle, M.Ott, C.Schmüser, A.Hartwig, V.Danilov, H.-E.Wagner, J.Meichsner, Macromol.Mat.Eng. 297, 1091-1101 (2012).
(4) Electron induced functionalization of diamond by small organic groups;
A.Lafosse, M.Bertin, D.Caceres, C.Jäggle, P.Swiderek, D.Pliszka, and R.Azria, Eur.Phys.J. D 35, 363 (2005).