
Home | Group | Research | Publications | Seminars/Workshops | Teaching | Open Positions | How to find us | Intranet
Deutsch | English

Home | Group | Research | Publications | Seminars/Workshops | Teaching | Open Positions | How to find us | Intranet
Deutsch | English
Electron-induced reactions can also be used to functionalize a surface. Exposing a thin layer of acetonitrile (CH3CN) on a hydrogen-terminated diamond surface to electrons with energies as low as 2 eV leads to attachment of acetonitrile fragments to the surface. We suggest that this is accompanied by an activation of the surface by atomic hydrogen released upon electron attachment.
| A Diamond surface is cooled to 35 K. | 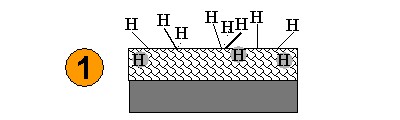 |
| 1-2 monolayers of acetonitril are condensed onto the surface and exposed to electrons. | 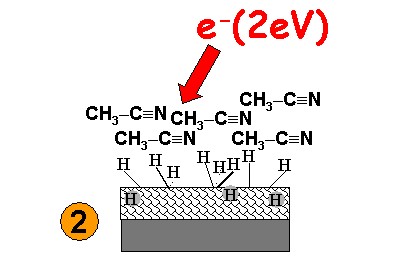 |
| The sample is heated to 400 K to evaporate physisorbed material. | 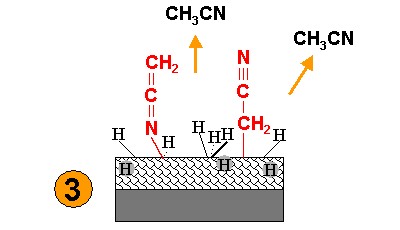 |
| The sample is cooled again to 35 K for recording of HREELS. | 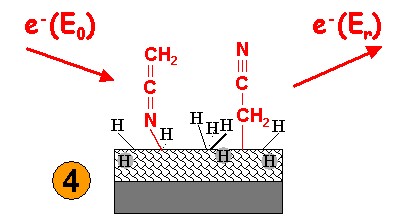 |
Evidence for the functionalization is obtained by HREEL spectra in the range of vibrational excitations. Besides other characteristic signals, acetonitril exhibits a C≡N stretching band. After exposure to electrons and desorption of the physisorbed material, the C≡N signal prevails. In addition, a band appears in the range of C=C and C=N vibrations. This is ascribed to the attachment of molecular fragments in two different geometries.
More information:
Electron induced functionalization of diamond by small organic groups;
A.Lafosse, M.Bertin, D.Caceres, C.Jäggle, P.Swiderek, D.Pliszka, and R.Azria, Eur.Phys.J. D 35, 363 (2005).
A new strategy to control chemical synthesis by the use of low-energy electron exposure relies on the electrostatic attraction caused by soft ionization of one of the reaction partners. This approach, which is complementary to the strategy of controlling chemical reactions by inducing DEA, has been used to synthesize aminoethane. This and similar reactions are expected to open new strategies for surface functionalization.
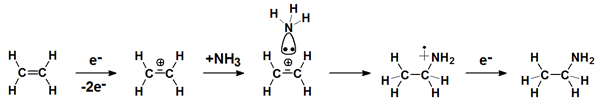
The reaction is supposed to proceed as follows: Ionisation above the first threshold removes an electron from the π orbital of C2H4. The cation interacts attractively with the lone pair of NH3. Alternatively, ionisation can also occur from the lone pair of NH3 and the resulting cation be attracted towards the electron rich double bond of C2H4. Intramolecular H migration and subsequent neutralisation of the resulting adduct by thermalised electrons within the film then produces aminoethane (C2H5NH2). The reaction resembles a hydroamination except that the electron beam replaces the catalyst of the organic synthesis. Ionisation cirumvent the electrostatic repulsion that normally prevents the reaction between neutral C2H4 and NH3.
More information:
Low-Energy-Electron-Induced Hydroamination of an Alkene;
T.Hamann, E.Böhler, P.Swiderek, Angew.Chem.Int.Ed. 48, 4643 (2009).