
Home | Group | Research | Publications | Seminars/Workshops | Teaching | Open Positions | How to find us | Intranet
Deutsch | English

Home | Group | Research | Publications | Seminars/Workshops | Teaching | Open Positions | How to find us | Intranet
Deutsch | English
Prototypical organic molecules are investigated at electron energies below 20 eV to learn about the mechanisms of electron-induced reactions. These energies are characteristic of secondary electrons formed in abundance under exposure of a material to higher-energy radiation and thus are also the most important initiators of chemical reactions in technical processes. On the other hand, it is known that at electron energies below the ionization threshold electron attachment can lead to efficient fragmentation of a molecule. This should provide new opportunities to control the modification of a material.
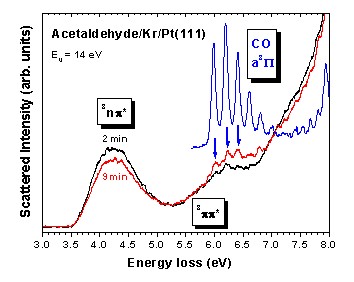
High-resolution electron-energy-loss (HREEL) spectra within the range of electronic excitations show that acetaldehyde (CH3CHO), similar to other oxygen-containing molecules, yields CO under exposure to low-energy electrons (Experiment).
This leaves open the question about products resulting from the remaining hydrocarbon fragments. Thermal desorption spectrometry (TDS, Experiment) shows that besides CO, that desorbs first upon increase of the sample temperature, CH4 (mass 16) and CH3CH2CHO (mass 58) are formed.
More information:
Fate of reactive intermediates formed in acetaldehyde under exposure to low-energy electrons;
P.Swiderek, C.Jäggle, D.Bankmann, E.Burean, J.Phys.Chem.B 111, 303 (2007).
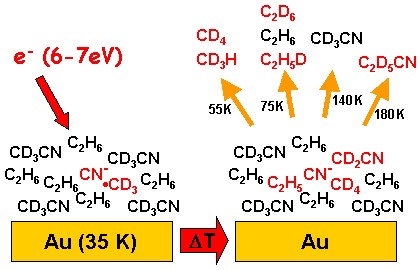
Experiments using mixtures of two different molecules show which conditions are required to induce reactions between the two partners. The results demonstrate wether electron-induced reactions can be used to functionalize simple starting materials. Electron attachment at 7 eV initiates a reaction yielding C2D6 (mass 36) in a mixture of deuterated acetonitrile (CD3CN) and ethane (C2H6). Other products (CD4, CD3H, C2H5D und C2D5CN) give evidence of intermolecular reactions. Above the ionization threshold, a reaction between fragments of the two substances yields C2H5CD3.
More information:
Electron-induced reactions in condensed films of acetonitrile and ethane;
I.Ipolyi, W.Michaelis, P.Swiderek, PCCP 8, 182 (2007).