
Home | Group | Research | Publications | Seminars/Workshops | Teaching | Open Positions | How to find us | Intranet
Deutsch | English

Home | Group | Research | Publications | Seminars/Workshops | Teaching | Open Positions | How to find us | Intranet
Deutsch | English
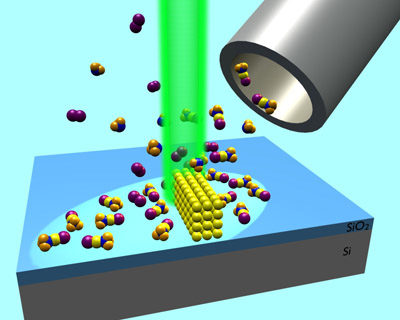

Electron-induced chemistry is a versatile approach to the fabrication of nanoscale materials and devices. Depending on the electron source used in such processes, different types of nanostructures are accessible. Using a tightly focused beam, structures of arbitrary shape with dimensions in the nanometer regime can be directly written on surfaces. This so-called focused electron beam induced deposition (FEBID) produces solid materials through electron-induced decomposition of volatile precursors such as metal organic compounds.
In contrast, divergent lower-energy electron beams process surfaces on macroscopic length scales. In this case, patterns can be imprinted onto a surface by electron exposure through a mask. Such patterns themselves often consist of smaller structures, namely, when electron exposure leads to formation of nanoparticles in the irradiated surface area. Hierarchical surface patterns are thus accessible.
Our research aims at a detailed understanding of chemistry underlying the fabrication of nanoscale materials by processes utilizing electron beams. Such processes are commercially relevant in areas such as the repair of masks for photolithography. Our activities are part of an international network developed through the previous European COST Action CELINA (Chemistry for ELectron-Induced NAnofabrication) coordinated by us and extended within the Marie-Curie Innovative Training Network ELENA (Low energy ELEctron driven chemistry for the advantage of emerging NAnofabrication methods) . A few results are highlighted here.
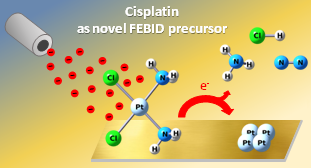
Electron-induced fabrication processes often do not yield a pure material. Instead, significant amounts of unwanted contaminations are embedded in the deposits. For instance, halide ligands are difficult to remove and thus require extensive irradiation. However, desorption of Cl ligands is enhanced in presence of NH3 because HCl is formed. Therefore, cisplatin decomposes under electron irradiation to yield pure platinum making it an attractive alternative to established FEBID precursors for platinum deposition.
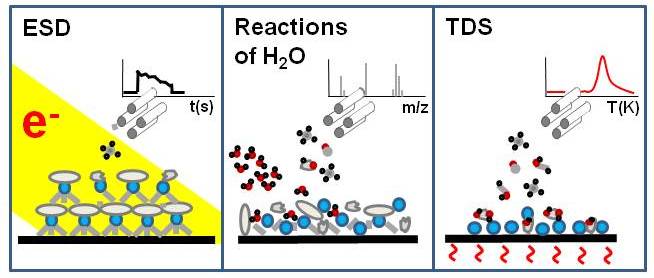
Water vapour in combination with electron irradiation is an efficient means to remove carbon from nanoscale materials. In such a process, water produces reactive species that can break down carbon residues and remove it primarily in form of carbon monoxide. We apply different types of desorption experiments in ultrahigh vacuum to unravel the fundamentals of this chemistry.
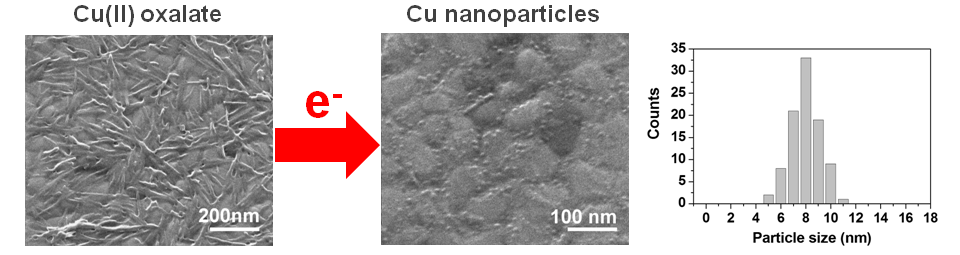
Electron-irradiation transforms surface grown metal-organic layers into a variety of nanostructured materials. For instance, copper(II) oxalate is converted to pure copper nanoparticles while the counterions are quantitatively removed as CO2. In contrast, surface layers of HKUST-1 yield copper nanoparticles embedded in an organic matrix, a material which can serve as template for autocatalytic decomposition of Fe(CO)5. Comparing HKUST-1 to copper(II) oxalate, we have started to unravel the fundamental chemistry of such surface activation by electron irradiation.
Electron-induced chemistry is also highly relevant to state-of-the-art nanolithography using extreme ultraviolet radiation (EUVL). Here, the energetic ionizing radiation with a wavelength of 13.5 nm releases photoelectrons and subsequently also secondary electrons in the resist layer. Using a novel Zn oxocluster resist material as example, it was shown during a collaborative project with the i>Advanced Research Center for Nanolithography in Amsterdam that these electrons are as efficient as the EUV photon itself in triggering the desired resist conversion. Therefore, their effect must be taken into account when developing new resists materials.
More information:
(1) Role of low-energy electrons in the solubility switch of Zn-based oxocluster photoresist for extreme ultraviolet lithography (2021 PCCP HOT article);
M. Rohdenburg, N. Thakur, R. Cartaya, S. Castellanos, P. Swiderek,
Phys. Chem. Chem. Phys. 23, 16646-16657 (2021).Edit-Stift
(2) Combined Ammonia and Electron Processing of a Carbon-Rich Ruthenium Nanomaterial Fabricated by Electron-Induced Deposition;
M. Rohdenburg, J. E. Fröch, P. Martinović, C. J. Lobo, P. Swiderek,
Micromachines 11, 769 (2020).
(3) Water-Assisted Process for Purification of Ruthenium Nanomaterial Fabricated by Electron Beam Induced Deposition;
M. Rohdenburg, R. Winkler, D. Kuhness, H. Plank, P. Swiderek,
ACS Applied Nano Materials 3, 8352–8364 (2020).
(4) Efficient NH3-based process to remove chlorine from electron beam deposited ruthenium produced from η3-allyl ruthenium tricarbonyl chloride;
M. Rohdenburg, H. Boeckers, C. R. Brewer, L. McElwee-White, P. Swiderek,
Sci. Rep. 10, 10901 (2020).
(5) Focused electron beam-induced processing;
D.H. Fairbrother, S.G. Rosenberg, C.W. Hagen, I. Utke, P. Swiderek,
in Low-Energy Electrons: Fundamentals and Applications, O. Ingólfsson (Ed.), Pan Stanford (2019), pp. 219.
(6) Cisplatin as Potential Pt FEBID Precursor: NH3 Ligands Enhance the Electron-Induced Removal of Chlorine;
M. Rohdenburg, P. Martinovic, K. Ahlenhoff, S. Koch, D. Emmrich, A. Gölzhäuser, P. Swiderek
J. Phys. Chem. C 123, 21774-21787 (2019).
(7) Electron-induced chemistry of surface-grown coordination polymers with different linker anions;
K. Ahlenhoff, S. Koch, D. Emmrich, R. Dalpke, A. Gölzhäuser, P. Swiderek
Phys. Chem. Chem. Phys. 21, 2351-2364 (2019).
(8) Electron beam induced surface activation of the metal organic framework HKUST 1: Unravelling the underlying chemistry;
K. Ahlenhoff, C. Preischl, P. Swiderek, H. Marbach,
J. Phys. Chem. C 46, 26658-26670 (2018).
(9) Chemistry for electron-induced nanofabrication;
P. Swiderek, H. Marbach, C.W. Hagen,
Beilstein J. Nanotechnol. 9, 1317-1320 (2018).
(10) Electron-driven and thermal chemistry during water-assisted purification of platinum nanomaterials generated by electron beam induced deposition;
Z. Warneke, M. Rohdenburg, J. Warneke, J. Kopyra, P. Swiderek,
Beilstein J. Nanotechnol. 9, 77-90 (2018).
(11) Efficient electron-induced removal of oxalate ions and formation of copper nanoparticles from copper(II) oxalate precursor layers;
K. Rückriem, S. Grotheer, H. Vieker, P. Penner, A. Beyer, A. Gölzhäuser,
P. Swiderek, Beilstein J. Nanotechnol. 7, 852-861 (2016).
(12) The role of NH3 in the electron-induced reactions of adsorbed and solid cisplatin;
J. Warneke, M. Rohdenburg, Y. Zhang, J. Orzagh, A. Vaz, I. Utke, J.Th.M. De Hosson, W.F. van Dorp, P. Swiderek; J.Phys.Chem. C 120, 4112-4120 (2016).
(13) Acetone and the precursor ligand acetylacetone: Distinctly different electron beam induced decomposition?
J. Warneke, W. F. Van Dorp, P. Rudolf, M. Stano, P. Papp, S. Matejcik, T. Borrmann, P. Swiderek;
Phys.Chem.Chem.Phys. 17, 1204-1216 (2015).
(14) Fundamentals of interactions of electrons with molecules;
John H. Moore, Petra Swiderek, Stefan Matejcik and Michael Allan,
in Nanofabrication using focused ion and electron beams: Principles and applications, P. Russell, I. Utke and S. Moshkalev (Eds.), Oxford University Press, New York (2012), pp. 184.
(15) Low Energy Electron Induced Decomposition and Reactions of Adsorbed Tetrakis(trifluorophosphine)platinum (Pt(PF3)4); K.Landheer, S.G.Rosenberg, L.Bernau, P.Swiderek, I.Utke, K.Hagen, D.H.Fairbrother, J. Phys. Chem. C 115, 17452 (2011).
(16) Electron-induced reactions of MeCpPtMe3 investigated by HREELS;
M.N.Hedhili, J.H.Bredehöft, P.Swiderek; J. Phys. Chem. C 113, 13282 (2009).

COST Action CM1301 CELINA
( Chemistry for ELectron Induced NAnofabrication)